[最も好ましい] total yield formula chemistry 139438-How to calculate yield organic chemistry
Chemistry Is Everywhere Actual Yields in Drug Synthesis and Purification Many drugs are the product of several steps of chemical synthesis Each step typically occurs with less than 100% yield, so the overall percent yield might be very smallMole ratio MgSiO = 113 !142a Percentage yield of the product of a reaction Even though no atoms are gained or lost in a chemical reaction (law of conservation of mass), unfortunately it is not always possible to obtain the calculated amount of a product (ie 100% yield) because the reaction may not go to completion because it may be reversible or some of the product may be lost when it is separated from the

Reaction Yield An Overview Sciencedirect Topics
How to calculate yield organic chemistry
How to calculate yield organic chemistry-Obviously total conversion under ideal circumstances will be 100 per cent, but in reality, that will not happen The formula for the percentage yield calculation as found in the data booklet isIf every photon absorbed results in a photon emitted The maximum fluorescence quantum yield is 10, and compounds with quantum yields of 010 are still considered fluorescent Another way to define the fluorescence quantum yield is by the excited state decay rates \ \Phi = \dfrac{k_f}{\sum_i k_i} \label{Eq1}\
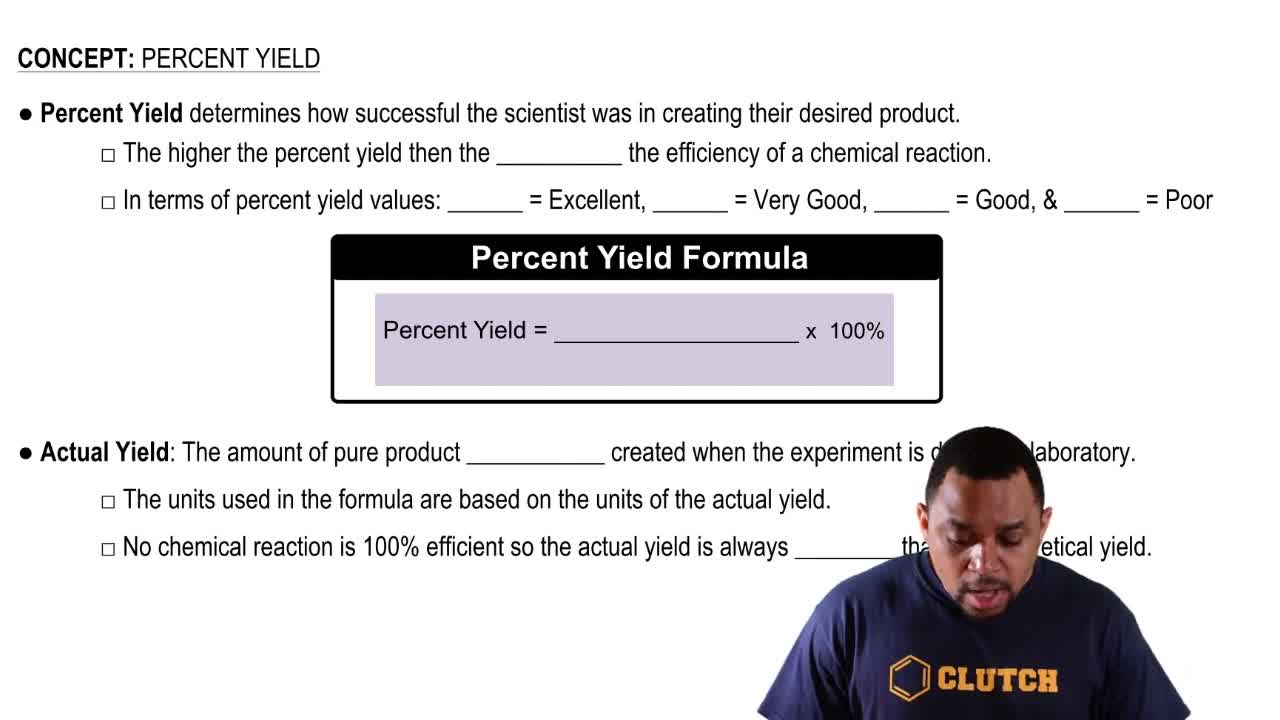


Percent Yield Chemistry Video Clutch Prep
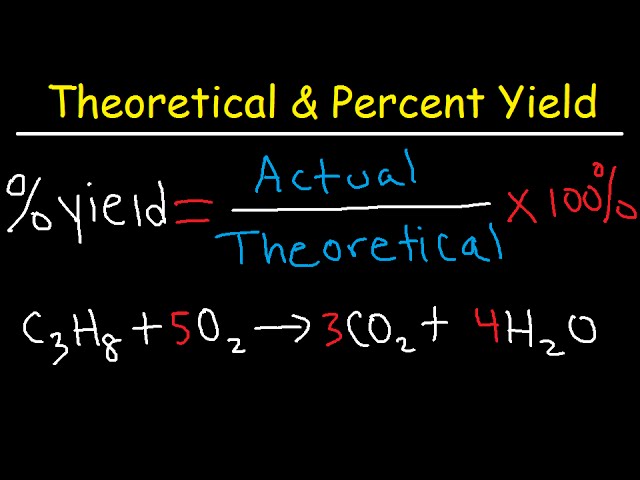
The dividend yield formula is as follows Dividend Yield = Dividend per share / Market value per share Where Dividend per share Dividend Per Share (DPS) Dividend Per Share (DPS) is the total amount of dividends attributed to each individual share outstanding of a company Calculating the dividend per share is the company's total annual dividend payment, divided by the total number of shares outstanding32 Determining Empirical and Molecular Formulas;In reality, most reactions are not perfectly efficient If you perform the experiment, you'll end up with a smaller amount, the actual yield To express the efficiency of a reaction, you can calculate the percent yield using this formula %yield = (actual yield/theoretical yield) x 100 A percent yield of 90% means the reaction was 90% efficient, and 10% of the materials were wasted (they failed to react, or their products were not captured)
Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Usually, you have to calculate the theoretical yield based on the balanced equation In this equation, the reactant and the product have a 11 mole ratio, so if you know the amount of reactant, you know the theoretical yield is the same value in moles (not grams!)Filter Yield, lbs/hr/sq ft = (Filter Operation, hr/day) (Area, sq ft) (Solids Loading, lbs/day) (Recovery, % / 100%) Flow Rate, cfs = (Area, sq ft) (Velocity, ft/sec) or Q = AV where Q = flow rate, A = area, V= velocity Food/Microorganism Ratio = MLVSS, lbs BOD5 , lbs/day Force, pounds = (Pressure, psi) (Area, sq in) Gallons/Capita/Day = Population31 Formula Mass and the Mole Concept;
Yield Burning The illegal practice of underwriters marking up the prices on bonds for the purpose of reducing the yield on the bond This practice, referred to as "burning the yield," is doneYield is the mass of product formed in a chemical reaction Actual yield is the mass of product formed in an experiment or industrial process Theoretical yield is the mass of product predicted by the balanced chemical equation for the reaction Percentage yield = (actual yield ÷ theoretical yield) × 10031 Formula Mass and the Mole Concept;
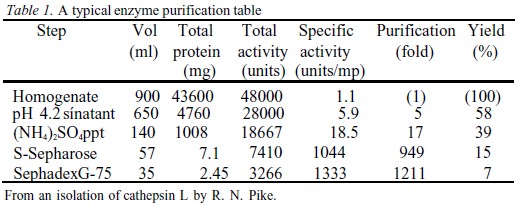


The Purification Table Recombinant Protein


3
EXAMPLE 1 WEIGHT PERCENT TO FORMULA Oxide wt % MolWt oxide Moles oxide Moles cation Mole oxygen SiO 2 5985 0996* 0996 2*0996 = 1992 MgO 4015 0996 0996 0996 Total 100 2998 * 5985/ !Formula MgSiO 3 enstatiteThen, yield is defined as "% of total starting protein/enzyme in final purified protein fraction" With this definition of yield, it would make sense to say that you get 30% yield in the second step since you have 3,000 mg / 10,000 mg of the original protein amount The definition of yield in my notes mentions nothing of activity



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



How To Calculate Percent Yield In Chemistry 15 Steps
Percentage Yield in a Snap!AP Chemistry Limiting Reactants and Percent Yield 1 Aluminum metal reacts with chlorine gas in a synthesis reaction a Write the balanced equation for this reaction b If 152 g of aluminum reacts with 391g of chlorine, identify the limiting reactant the molecular formula if the molar mass of the compound is 4406 g/mol?The total yield is the capital gain plus the annual dividend divided by the initial investment A capital gain is the profit from the sale of an asset (in this case, stock) To calculate the capital gain, subtract the ending price of the stock from the initial price The ending price in our example is $100



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com



4 4 Reaction Yields Chemistry
To find the percentage yield, you will need to divide the actual yield by the theoretical yield Then, multiply it by 100 ie % yield = actual yield/theoretical yield x 100 An actual yield is the amount of a substance produced in an actual laboratory experiment It is based on an actual physical measurement of a quantity The theoretical yield is the yield as is calculated on paper ie through calculationsHowever, the definitions of the total amount of reactant to form a product per total amount of reactant consumed is used (Definition 2) as well as the total amount of desired product formed per total amount of limiting reactant consumed (Definition 3) This last definition is the same as definition 1 for yield Batch or SemiBatch1 mole of salicylic acid gives 1 mole of aspirin So, 0725 moles gives 0725 moles of aspirin 0725 moles of aspirin = 0725 × 180 g = 1305 g So, the calculated mass of the reaction is 1305 g Step 4 Calculate the percent yield The actual mass obtained is 1212 g So, the percent yield = 1212 ÷ 1305 × 100% = 929% How to calculate the percent yield of a chemical reaction?



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Balancing Chemical Equations How To Balance Chemical Equations
Divide number of AI moles by two, since it takes four to make 2 AI2O3) Finally, to get a theoretical yield, you'll need to multiply the number of moles of the product by the molecular weight of the product (Ex 05 moles of AI2O3 multiplied by the molecular weight of AI2O3 is approximately grams per moleThe large number written in front of a chemical formula It shows the number of molecules answer choices Molecule Compound Yield sign Subscript Coefficient Chemical formula Yield sign alternatives At the end of chemical reactions, what is the total mass of the reactants compared to the total mass of the products?Ethanol 230 g ÷ 46 g/mol = 50 mol) Ethanol is used in a 25fold excess (50 mol ÷ mol) The theoretical molar yield is mol (the molar amount of the limiting compound, acetic acid)



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Percent Yield Percent Purity Solutions Examples Videos
Substitute the values in the corresponding formula Percentage yield = $\frac{Actual\;Determine the percentage yield Calculate the total percentage of the theoretical yield that was produced An actual yield is the exact yield of a substance generated from a chemical process Since no processFor example, for a synthesis like this A > B > C > D with yield of each step being AB 80 %, BC 50%, CD 50%, the overall yield of A > D will be 080*050*050 = %



Mass And Energy Balances
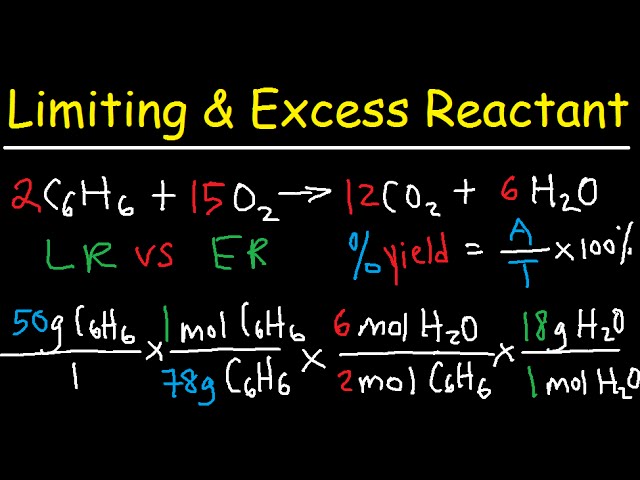


Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry Youtube
The percent yield of a given chemical process, on the other hand, evaluates the efficiency of a process by comparing the yield of product actually obtained to the maximum yield predicted by stoichiometry What is the total number ofThe following is the formula for calculating the actual yield of a process Y a = Y p / 100 * Y t Where Y a us the actual yield;Those are five of the reasons that many times a theoretical yield differs from the actual yield Remember, the theoretical yield is the amount of product produced when the entire limiting product is used up, but then actual yield is the amount of product that is actually produced in a chemical reaction



How To Calculate Percent Yield 3 Ways To Solve Chemistry Problems Tripboba Com
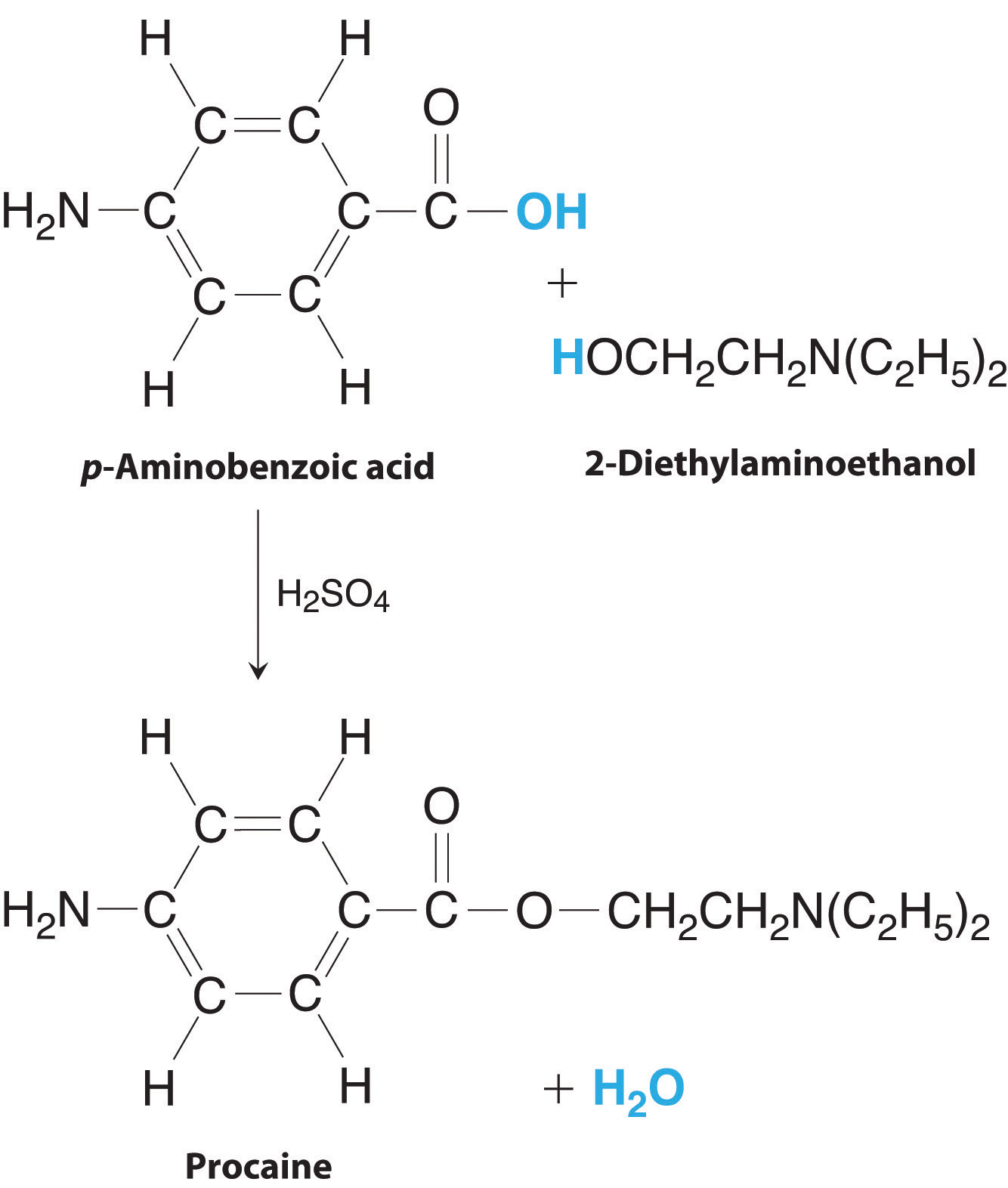


Mass Relationships In Chemical Equations
The percent yield of a given chemical process, on the other hand, evaluates the efficiency of a process by comparing the yield of product actually obtained to the maximum yield predicted by stoichiometry What is the total number ofSo, to stop you from wondering how to find theoretical yield, here is the theoretical yield formula mass of product = molecular weight of product * (moles of limiting reagent in reaction * stoichiometry of product)Percent Yield The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical equation, is called the theoretical yield of the reaction In practice, the amount of product obtained is called the actual yield, and it is often less than the theoretical yield for a number of reasons



Calculating Percent Recovery Percent Yield


Chemical Equations
Percent yield Refers to the efficiency of a chemical reaction;Most scientific papers have stated the formula for extraction yield calculation like this, The yield of extract (extractable components) expressed on dry weight basis of pulp was calculated fromTo calculate the theoretical yield, consider the reaction (2) CO ( g) 2 H 2 ( g) → CH 3 OH ( l) (3) 280 40 3 ( stoichiometric masses in g, kg, or tons) 12 t o n s H 2 × 3 C H 3 O H 40 H 2 = 96 t o n s C H 3 O H Thus, the theoretical yield from 12 metric tons (12x10 6 g) of hydrogen gas is 96 tons



Reaction Yield An Overview Sciencedirect Topics



Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab
Unlock the full Alevel Chemistry course at http//bitly/2MdGoZJ created by Ella Buluwela, Chemistry expert at SnapReviseSnapReTotal yield (%) = (yield 1 st react × yield 2 nd react × , etc ) × 100 For example, in a chemical transformation composed of three reactions and with partial yields each of 25%, 50%, and 75%, respectively, to calculate the total yield the above equation is applied (expressing the partial yields to decimals), resulting the followingYield to Maturity This reflects the total return an investor receives by holding the bond until it matures A bond's yield to maturity, or YTM, reflects all of the interest payments from the time of purchase until maturity, including interest earned on interest The formula for calculating YTM is as follows Let's work it out with an



How To Calculate Percent Yield In Chemistry 15 Steps



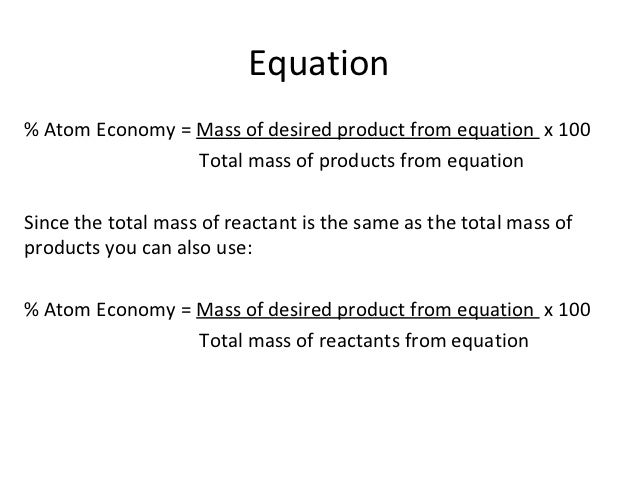
Atom Economy Wikipedia
Most scientific papers have stated the formula for extraction yield calculation like this, The yield of extract (extractable components) expressed on dry weight basis of pulp was calculated fromBefore performing chemical reactions, it is helpful to know how much product will be produced with given quantities of reactants This is known as the theoretical yieldThis is a strategy to use when calculating the theoretical yield of a chemical reactionThe question reads that the actual yield from 100 grams of Calcium Hydroxide is 50 grams of Calcium Oxide Hence, the percent yield is given by Percent Yield = Actual Yield/Theoretical Yield x



Green Chemistry For Chemical Synthesis Pnas


Q Tbn And9gctje7pkewyqa0nnrn06zpgzepy Yorrmegghf1btx8a49kkxzp8 Usqp Cau
The formula for calculating the percent yield is Percentage yield = mass of actual yield ÷ mass of theoretical yield × 100% Let's assume that you obtained an actual yield of 850 grams Then, the percent yield would be Percentage yield of NaCl = 850 grams ÷ 993 grams × 100% Percentage yield of NaCl = 8559%Volume = 05 × 24 = 12 dm 3 Remember that 1 dm 3 = 1 000 cm 3 so the volume is also 12 000 cm 3 The equation can be rearranged to find the number of moles, if the volume of gas at rtp is knownThe yield was 75% The molar amount of the reactants is calculated from the weights (acetic acid 1 g ÷ 60 g/mol = mol;



Reaction Yield An Overview Sciencedirect Topics


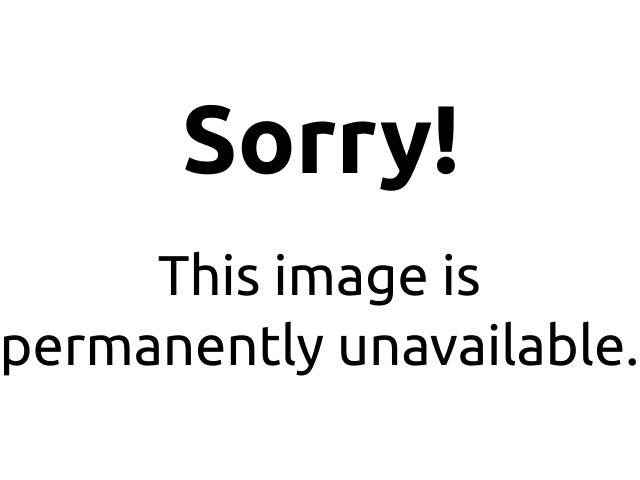
Calculating Percentage Yield
Chemists have to be concerned with just how completely their reactants react to form products To compare the amount of product obtained from a reaction with the amount that should have been obtained, they use percent yield You determine percent yield of a chemical reaction with the following formula Lovely, but what is an actualIf the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100It is the theoretical yield However, it is impossible for a ration to give 100 percent yield Because there is always some loss of reactant due to different factors Formula You can also find the theoretical yield yourself using the theoretical yield formula if percentage yield or actual yield is known Theoretical yield = Actual yield 100



Limiting Reactant And Reaction Yields Article Khan Academy



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com
Defined as the latex\frac{\mbox{actual yield}}{\mbox{theoretical yield}} \times 100/latex theoretical yield The amount of product that could possibly be produced in a given reaction, calculated according to the starting amount of the limiting reagentDefinition of Conversion, Selectivity and Yield This is the definition that we used in our simulation workYield}$$\times$ 100% Percentage yield = $\frac{06}{14}$$\times$ 100% Percentage yield = 429% The percentage yield of this reaction is 429%, Scientist tries to choose reactions with a high percentage yield or high atom economy



Percent Yield Chemistry Video Clutch Prep
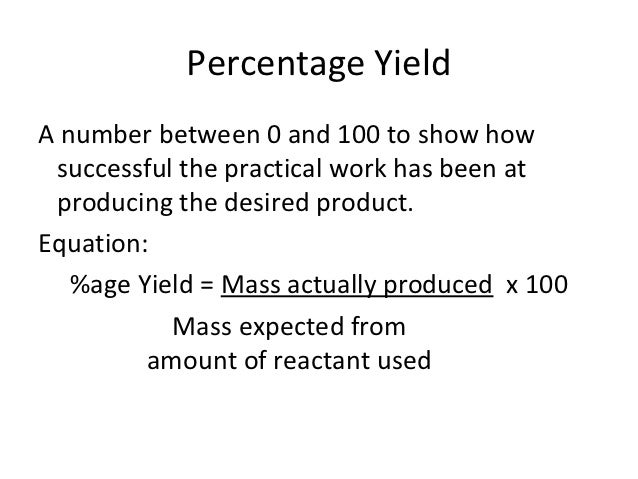


Chemistry Calculations Percent Yield And Atom Economy
32 Determining Empirical and Molecular Formulas;142a Percentage yield of the product of a reaction Even though no atoms are gained or lost in a chemical reaction (law of conservation of mass), unfortunately it is not always possible to obtain the calculated amount of a product (ie 100% yield) because the reaction may not go to completion because it may be reversible or some of the product may be lost when it is separated from theKey Difference – Percent Yield vs Percent Recovery Percent yield is the amount of a compound obtained from a chemical synthesis reaction with respect to the theoretically expected amount This is a percentage value It is used to determine the efficiency of a chemical reaction percent recovery is a term that is often used in organic chemistry regarding recrystallization processes



Why Is Yield In A Purification Table Measured Using Activity Instead Of Total Protein Chemistry Stack Exchange


Green Chemistry English Green Chemistry
Free Chemistry calculator Calculate chemical reactions and chemical properties stepbystep This website uses cookies to ensure you get the best experience By using this website, you agree to our Cookie PolicyHowever, the definitions of the total amount of reactant to form a product per total amount of reactant consumed is used (Definition 2) as well as the total amount of desired product formed per total amount of limiting reactant consumed (Definition 3) This last definition is the same as definition 1 for yield Batch or SemiBatch142a Percentage yield of the product of a reaction Even though no atoms are gained or lost in a chemical reaction (law of conservation of mass), unfortunately it is not always possible to obtain the calculated amount of a product (ie 100% yield) because the reaction may not go to completion because it may be reversible or some of the product may be lost when it is separated from the


Green Chemistry English Green Chemistry



Reaction Percent Yield Introduction And Practice Exercises
To use this formula for percent yield, you need to make sure that your actual yield and theoretical yield are in the same units If the actual yield is in grams, then theoretical yield also needsThe amount of product produced in a chemical synthesis is known as the reaction yield Typically, chemical yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity of product that could be produced based on the limiting reagentPercent Yield The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical equation, is called the theoretical yield of the reaction In practice, the amount of product obtained is called the actual yield, and it is often less than the theoretical yield for a number of reasons
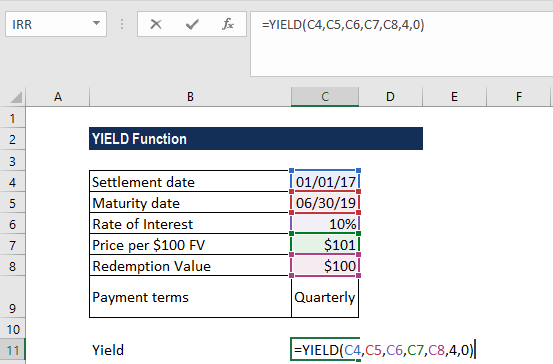


Yield Function Formula Examples Calculate Yield In Excel
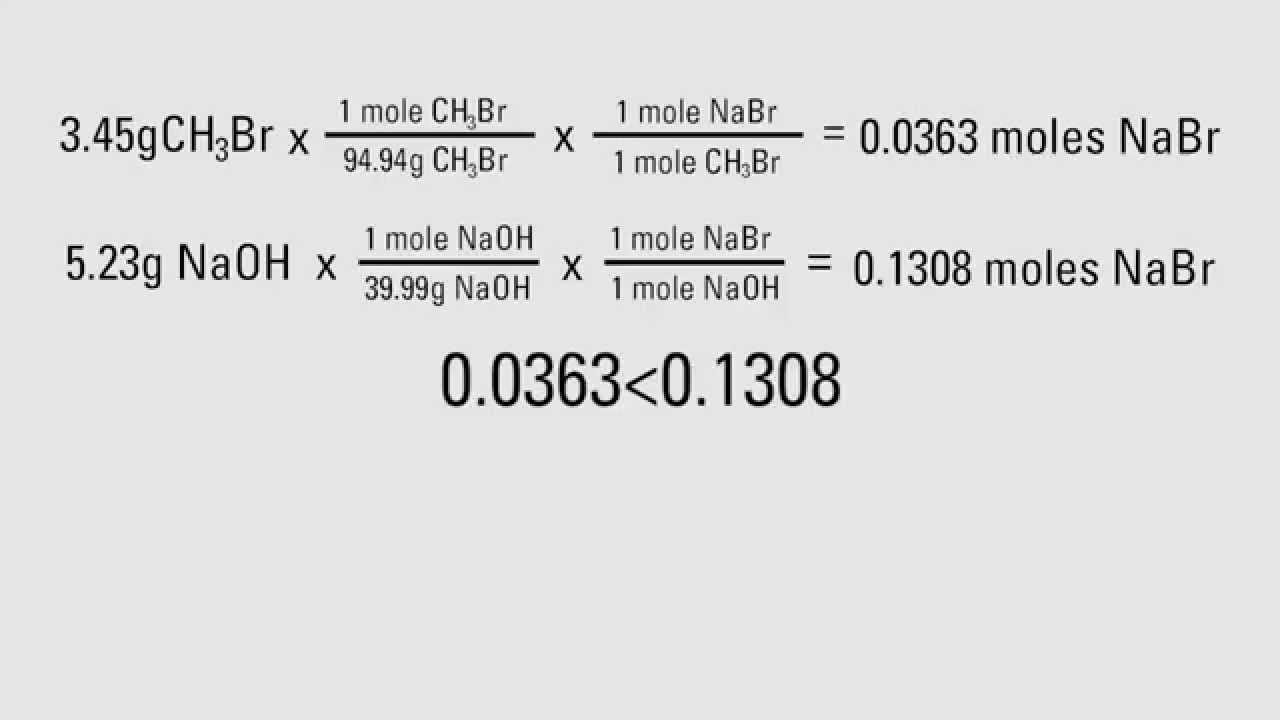


How To Calculate Theoretical Yields Youtube



Solved Report Sheet Experiment Chemical Reactions Of Copp Chegg Com



How To Calculate Percent Yield In Chemistry 15 Steps


Howto How To Find Percentage Yield In Chemistry



How To Calculate Percent Yield In Chemistry 15 Steps



How To Calculate Percent Yield In Chemistry 15 Steps


How To Calculate Theoretical Yield And Percent Yield Youtube



How To Calculate Percent Yield In Chemistry 15 Steps
/scientist-pouring-iron-chloride-into-beaker-of-potassium-thiocyanate-702545775-59fb5991845b340038498040.jpg)


What Is The Theoretical Yield Of A Reaction


Chemistry Atom Economy And Percentage Yield
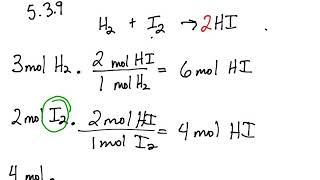


5 3 Calculating Reaction Yields Problems Chemistry Libretexts



How To Calculate Percent Yield Of A Chemical Reaction Youtube



Stoichiometric Calculations Quantitative Aspects Of Chemical Change Siyavula



Percent Yield Percent Purity Solutions Examples Videos


Mole Calculations Cheat Sheet By Fongrsy Download Free From Cheatography Cheatography Com Cheat Sheets For Every Occasion



Mock Examination 1 Answer Key


Green Chemistry English Green Chemistry
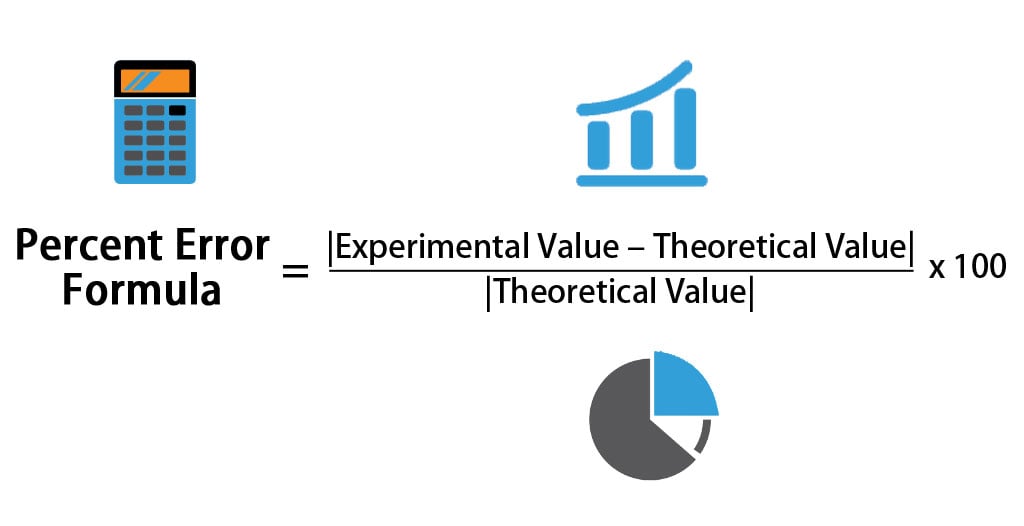


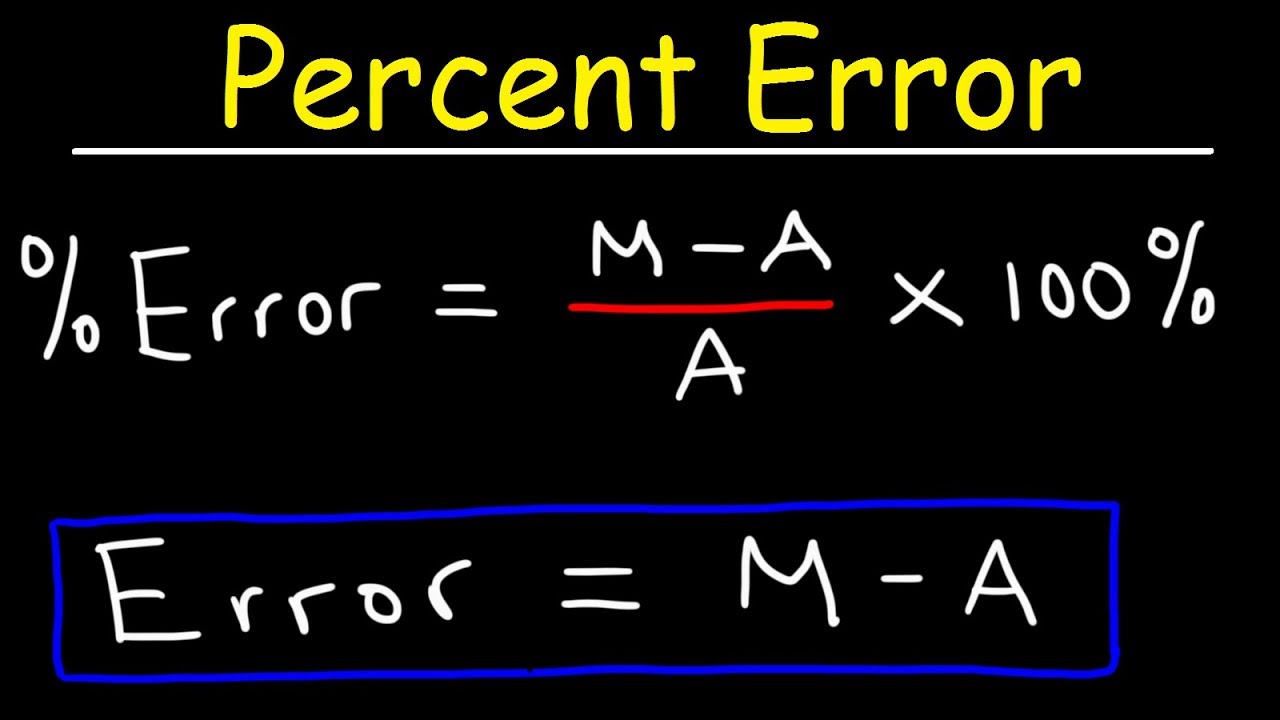
Percent Error Formula Calculator Excel Template



Quantitative Chemistry Secondary Science 4 All



Conversion Chemistry Wikipedia
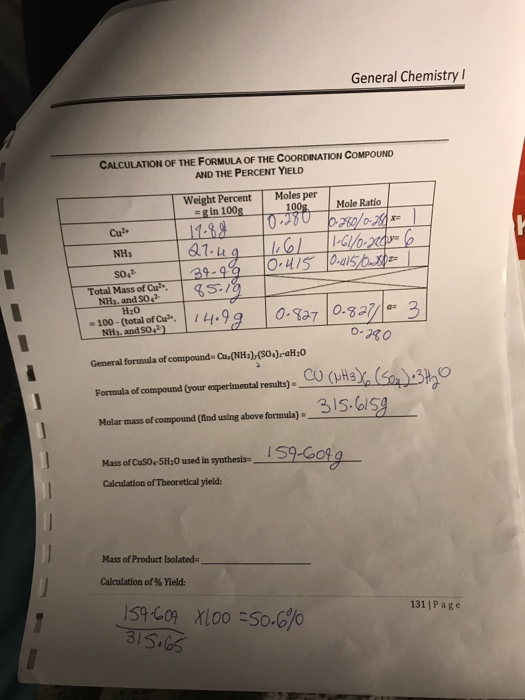


Solved Given All This Info What S The Theoretical Yield A Chegg Com
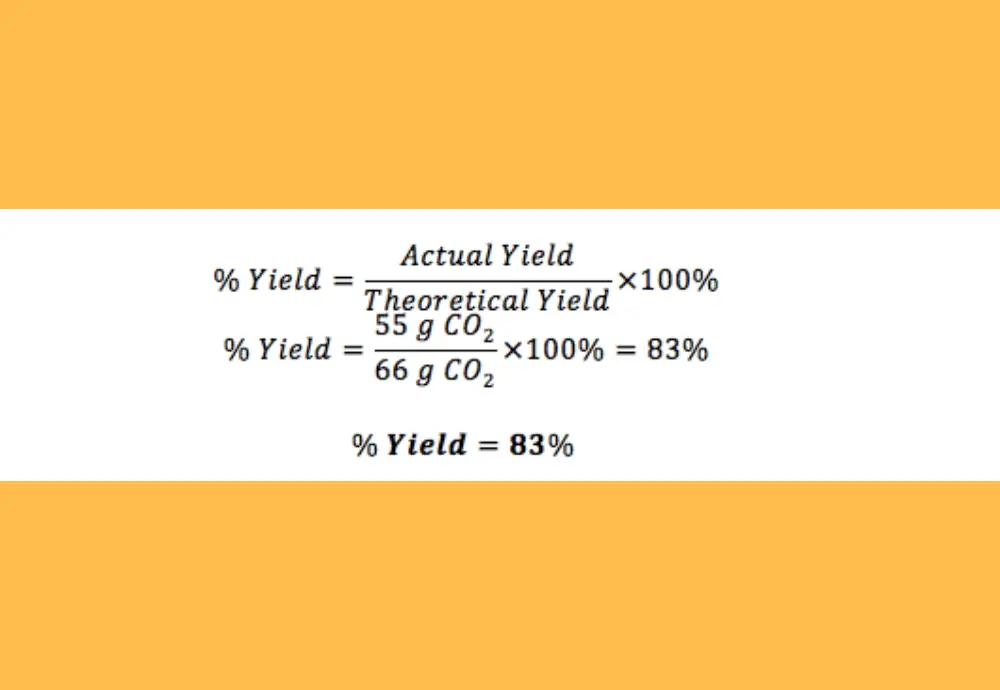


Percent Yield Calculator 100 Free Calculators Io


Chapter 6 Summary Notes
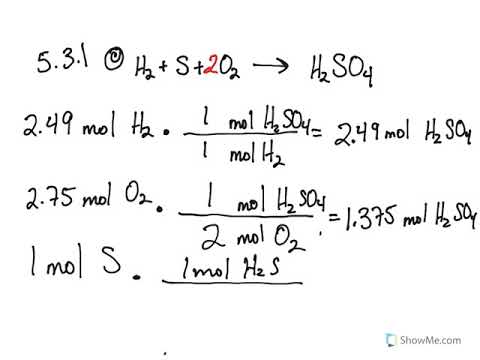


5 3 Calculating Reaction Yields Problems Chemistry Libretexts


Calculation Of Theoretical Yield Organic Chemistry I 212 01


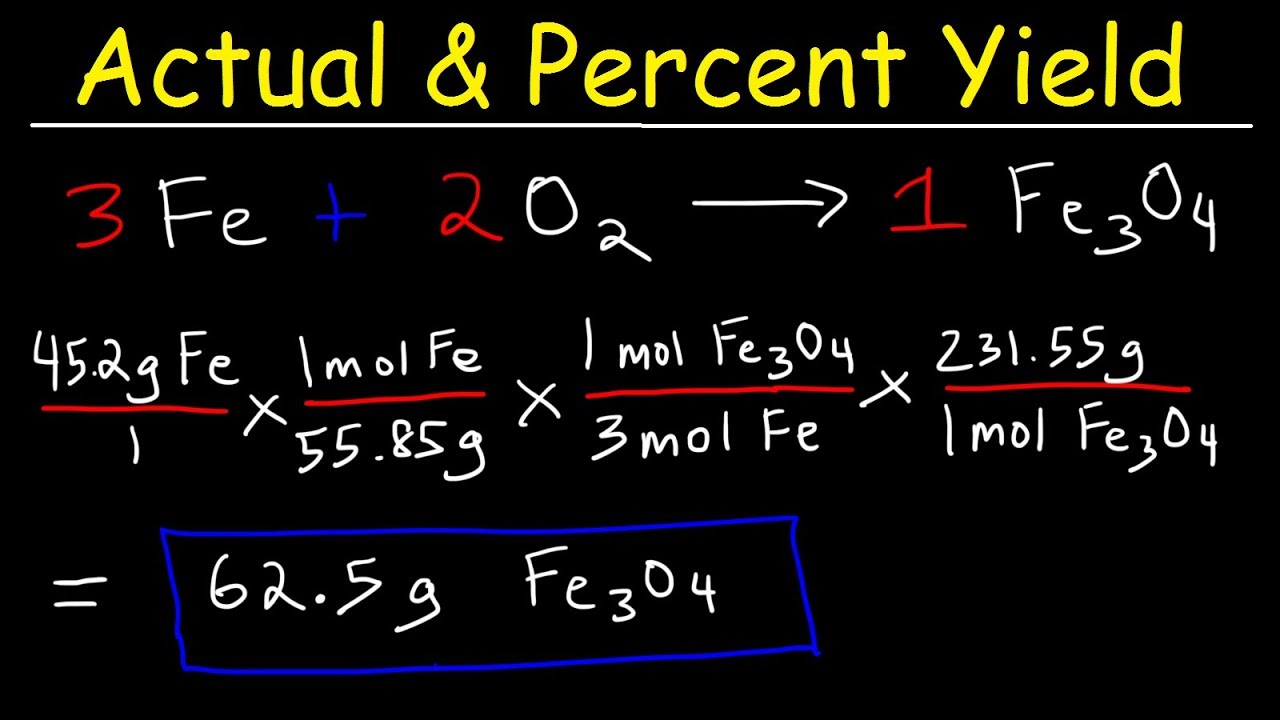
How To Calculate Theoretical Yield And Percent Yield Youtube



How To Calculate Theoretical Yield 12 Steps With Pictures



Chemistry Calculations Percent Yield And Atom Economy


Q Tbn And9gcsoowhfh Ellws R7fhtegyfftxnyz 7tf9b1pxdba108ocwhdh Usqp Cau


How To Calculate The Percent Yield And Theoretical Yield Youtube



How To Calculate Percent Yield In Chemistry 15 Steps
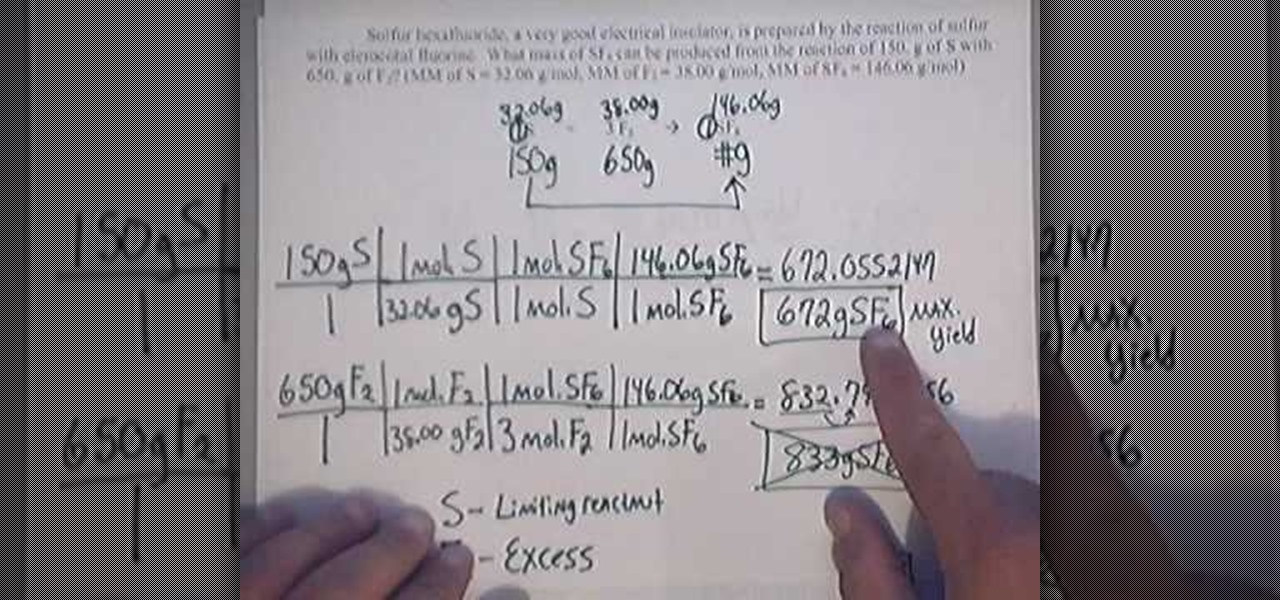


How To Calculate Percent Yield Math Wonderhowto
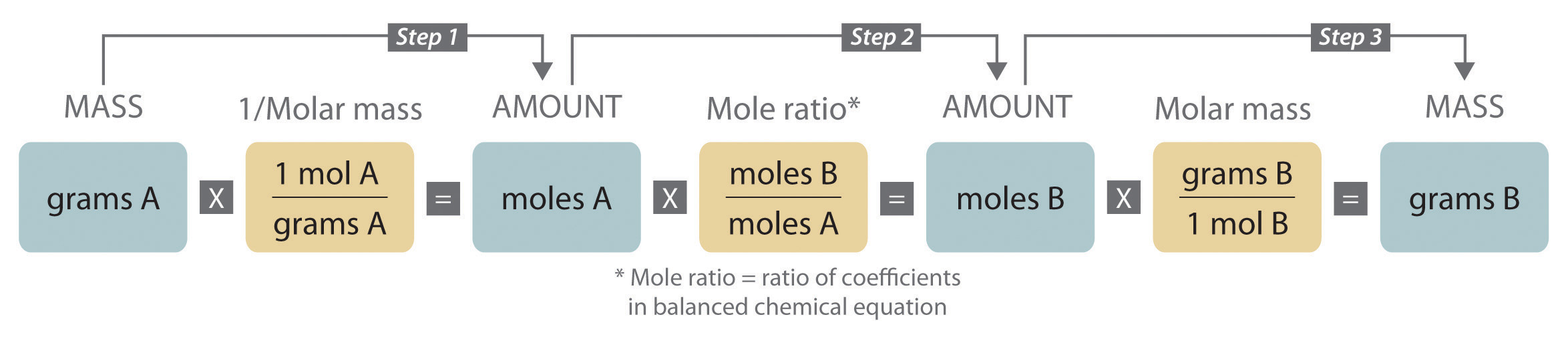


Mass Relationships In Chemical Equations



Magnesium Oxide Percent Yield Lab Report Schoolworkhelper



Percent Yield Calculator


Chemistry Atom Economy And Percentage Yield
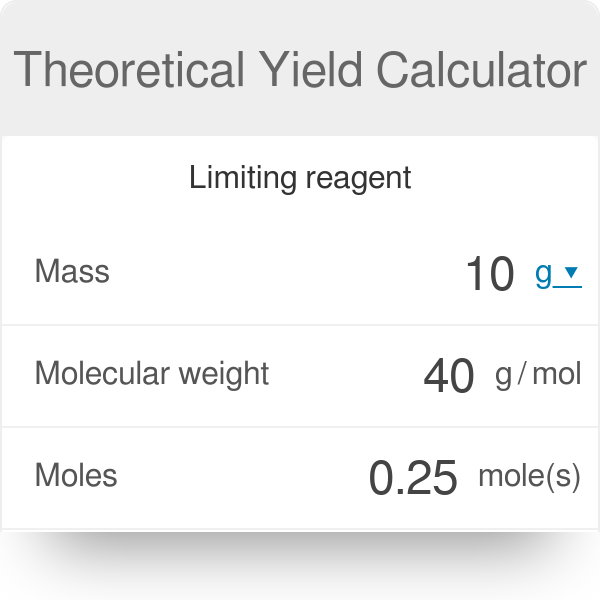


Theoretical Yield Calculator



Theoretical Actual Percent Yield Error Limiting Reagent And Excess Reactant That Remains Youtube



Solved Y Given The Followme Information Complete The Chem Chegg Com



How To Calculate Percent Yield In Chemistry 15 Steps



Calculating Amounts Of Reactants And Products Worked Example Video Khan Academy



Gcse Science Revision Chemistry Calculating Percentage Yield 1 Triple Youtube



Yield Calculations Faculty Staff Sites


8 4 Limiting Reactant And Theoretical Yield Chemistry Libretexts



Reaction Percent Yield Introduction And Practice Exercises



Percentage Yield Lab Answers Schoolworkhelper



Reaction Yield An Overview Sciencedirect Topics



Crop Yield Rice Equivalent Yield Total System Productivity And Download Table



Percent Yield Percent Purity Solutions Examples Videos


5 3 Calculating Reaction Yields Problems Chemistry Libretexts



How To Calculate Percent Yield In Chemistry 15 Steps



How To Do Percentage Yield Calculations How To Calculate Percent Yield Of A Chemical Preparation Reasons Why Don T You Get 100 Yield Gcse Chemistry Igcse Ks4 Science A Level Gce As
/148302528-56a12f323df78cf77268383a.jpg)


Percent Yield Definition And Formula



Chemical Equations Protocol



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com
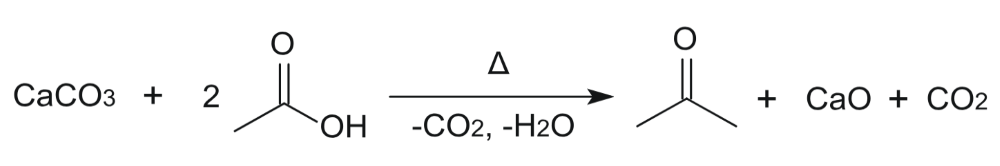


Percent Yield Calculator


Q Tbn And9gcrynatxciaobbvt Edbzcqmlgnhpv Acw5cqav2pwzm1fudi Ih Usqp Cau



How To Calculate Percent Yield In Chemistry 15 Steps



How To Find Actual Yield Theoretical Yield And Percent Yield Examples Practice Problems Youtube
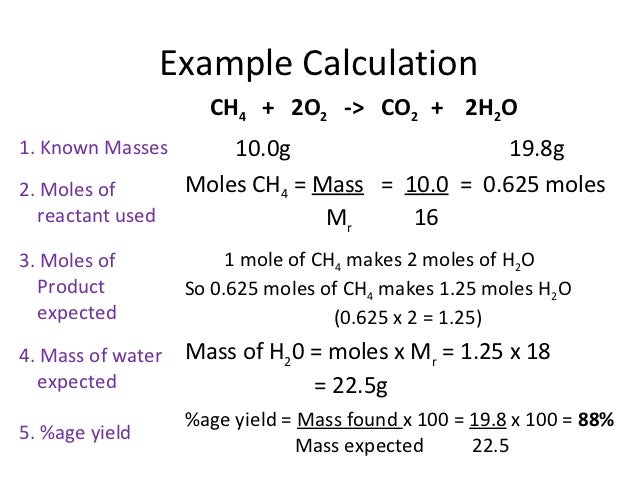


Chemistry Calculations Percent Yield And Atom Economy



How To Calculate Percent Yield In Chemistry 15 Steps
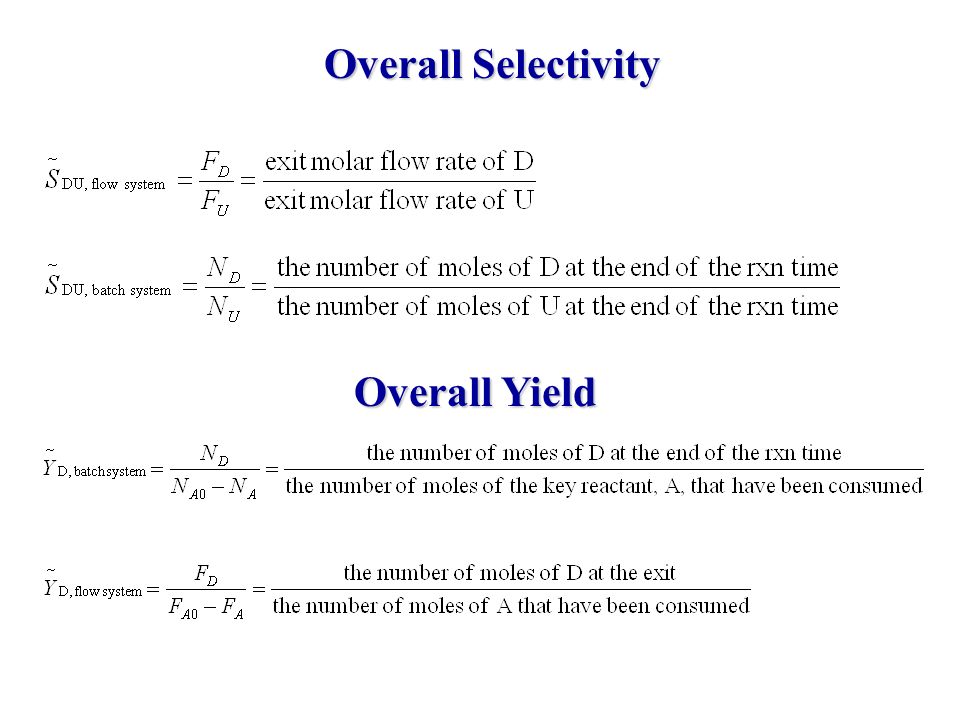


Chapter 6 Chemical Reaction Engineering Mutiple Reactions Ppt Video Online Download



How To Calculate Percent Yield In Chemistry 15 Steps


5y1 Org Download 2e0ffc2bd49eef3f0cbd Pdf


Www Mtsu Edu Chemistry Chem1010 Pdfs Chapter 7calculations with chemical reactions Pdf


Chemistry Atom Economy And Percentage Yield



Reaction Stoichiometry Boundless Chemistry


Calculation Of Theoretical Yield Organic Chemistry I 212 01



How To Calculate Percent Yield In Chemistry 15 Steps



Solved What Is The Chemical Equation Once Balanced Full Chegg Com



Percent Yield Calculator



コメント
コメントを投稿